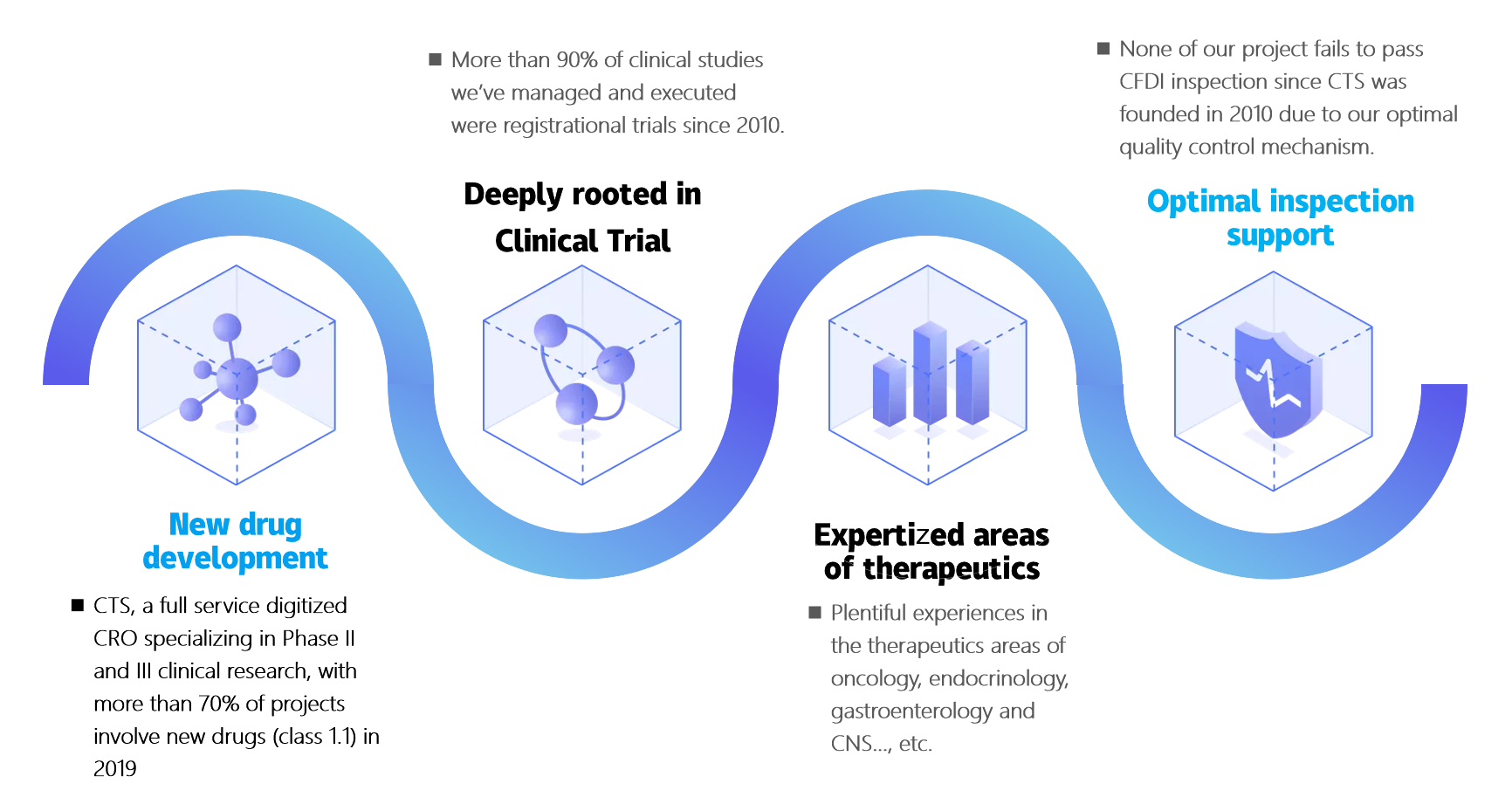
CRO - Digitized Clinical Trial Outsourcing Service
Applying the integrated cloud platform,from study execution, quality management to risk control for clinical trials, we are responsible for data quality of the study. We are your best partner providing clinical trial solutions with low-risk, low-cost and high-value in China.